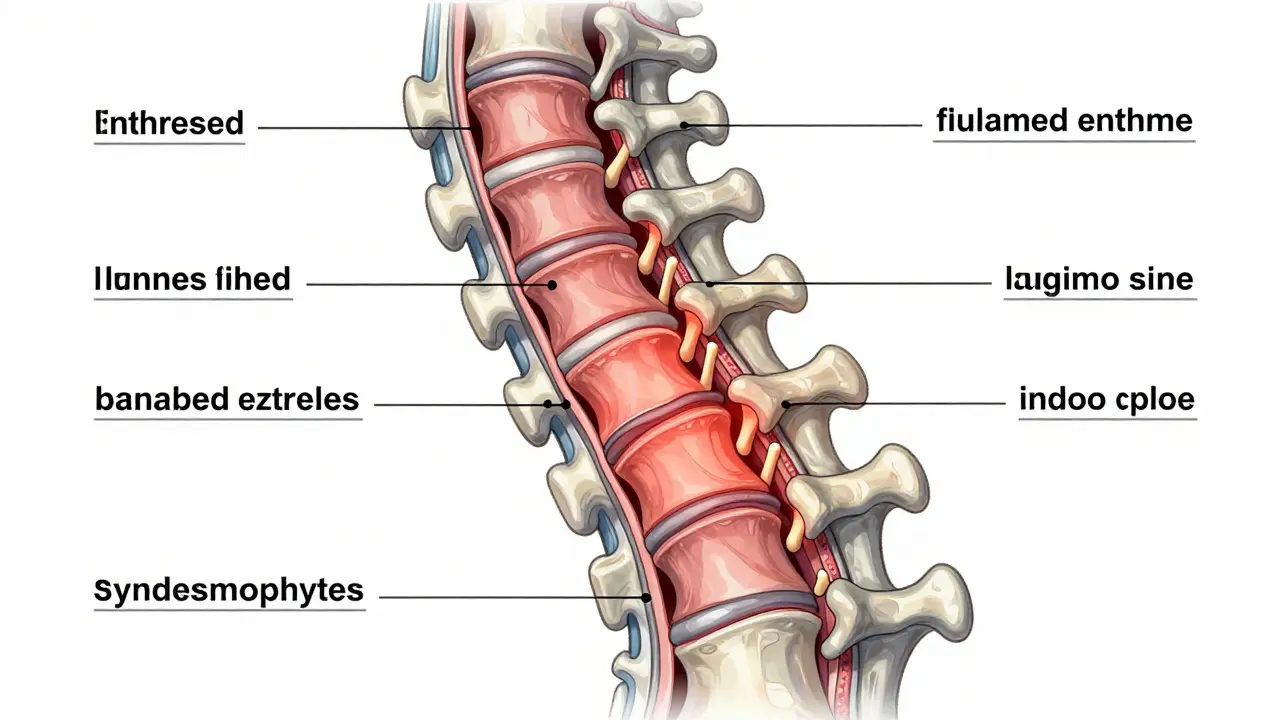
Ankylosing spondylitis is a chronic inflammatory disease that stiffens the spine. Learn how early diagnosis, targeted medication, and daily movement can prevent fusion and preserve mobility for life.
Generic and brand-name medications carry the same risk of drug interactions because they contain identical active ingredients. Scientific evidence confirms their safety and effectiveness, debunking common myths about generics.
Mixing sedatives like opioids, benzodiazepines, or alcohol can cause life-threatening respiratory depression. Learn how even prescribed drugs can become deadly when combined - and what you can do to stay safe.
Learn how centralized pharmacy systems prevent dangerous medication errors in seniors managing multiple pharmacies and prescribers. Real-world data, top platforms, and step-by-step safety strategies.
Medical weight management combines clinics, medications like semaglutide and tirzepatide, and ongoing monitoring to treat obesity as a chronic disease. With proven results, better outcomes than commercial programs, and growing access, it’s changing how we approach weight loss for good.

Authorized generics let brand manufacturers sell their own drugs under generic labels after patent expiry. With new FDA rules and falling delays in launches, they’re becoming a faster, more transparent way to lower drug prices - not just a tactic to block competition.

When your insurance denies coverage for a generic medication, you still have options. Learn how to file a legally protected exception request, what your doctor must include, how to cut costs, and why 58% of denials are overturned.
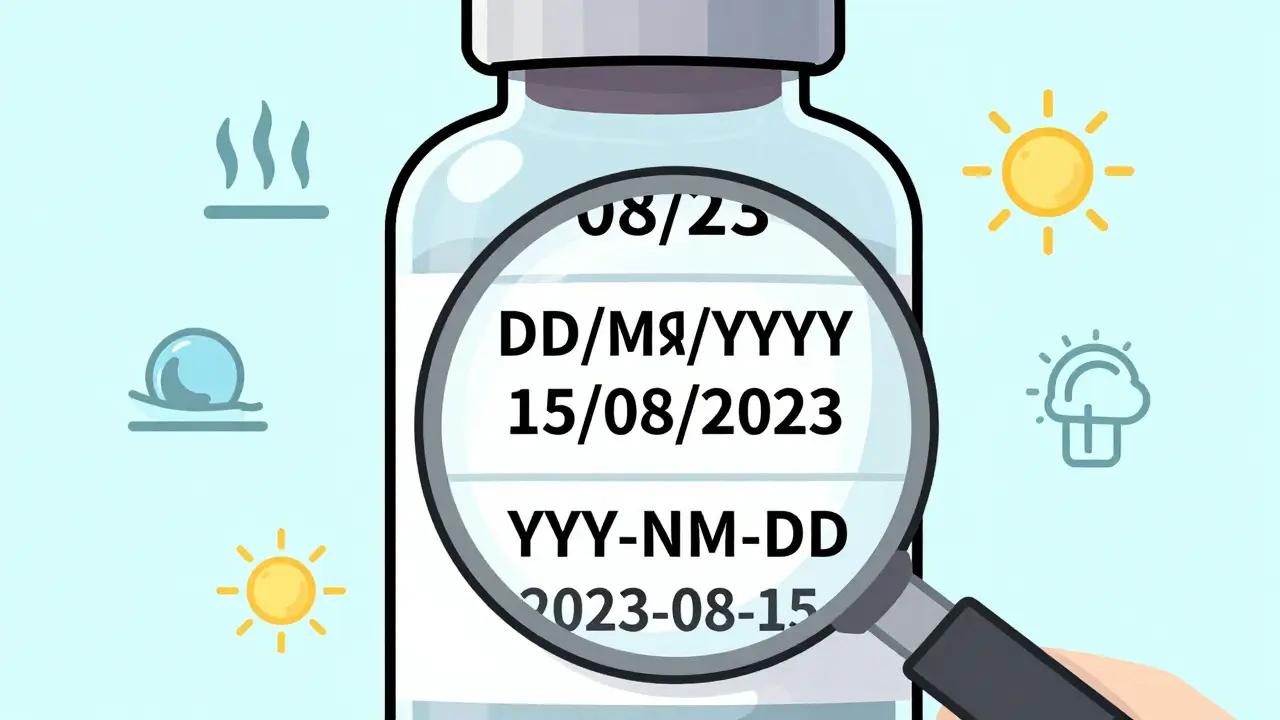
Learn how to read expiration dates on medicine packaging, understand the difference between pharmacy and manufacturer dates, and know which medications are risky to use after they expire. Stay safe with clear, practical advice.

Learn how to request lower-cost therapeutic alternatives for your medications. Discover practical steps, real examples, and how to navigate insurance hurdles to save money without compromising health.

Learn how to safely remove personal data from prescription bottles to prevent identity theft. Discover the best methods, common mistakes, and expert tips for secure disposal.