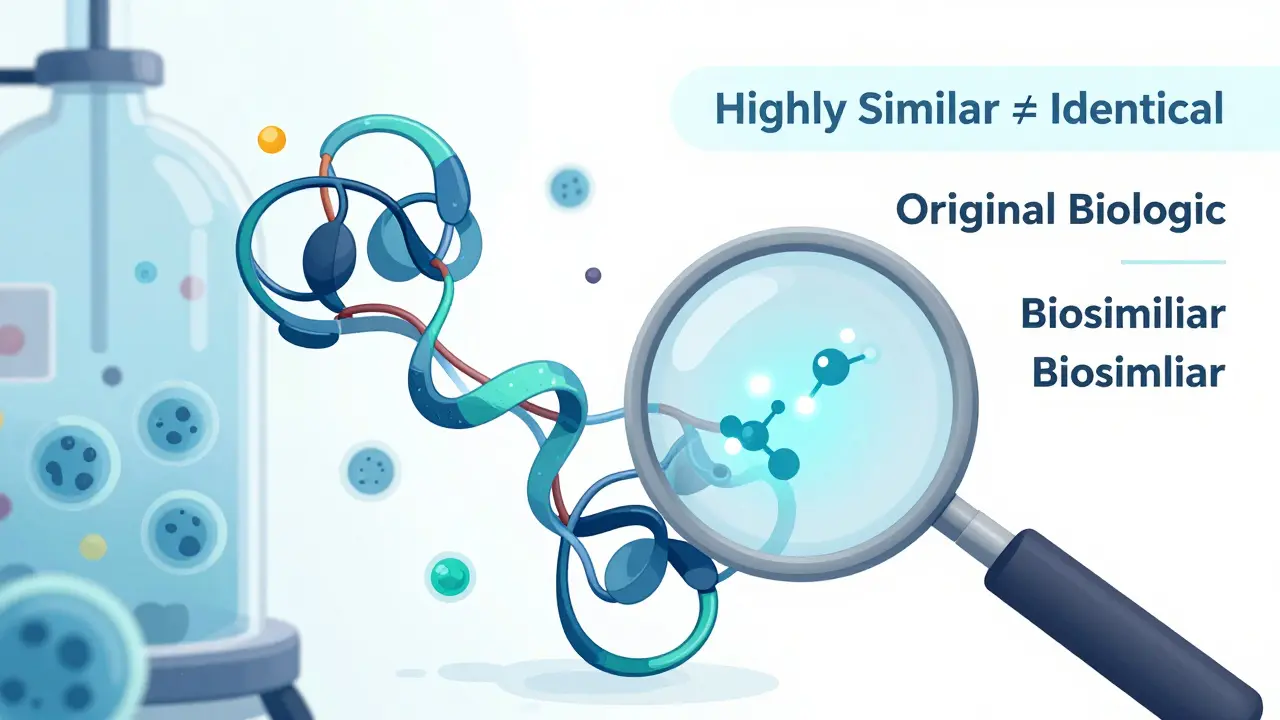
Monoclonal antibody biosimilars offer proven, cost-effective alternatives to expensive biologic drugs for cancer and autoimmune diseases. Learn which ones are approved, how they work, and why they’re changing patient care.
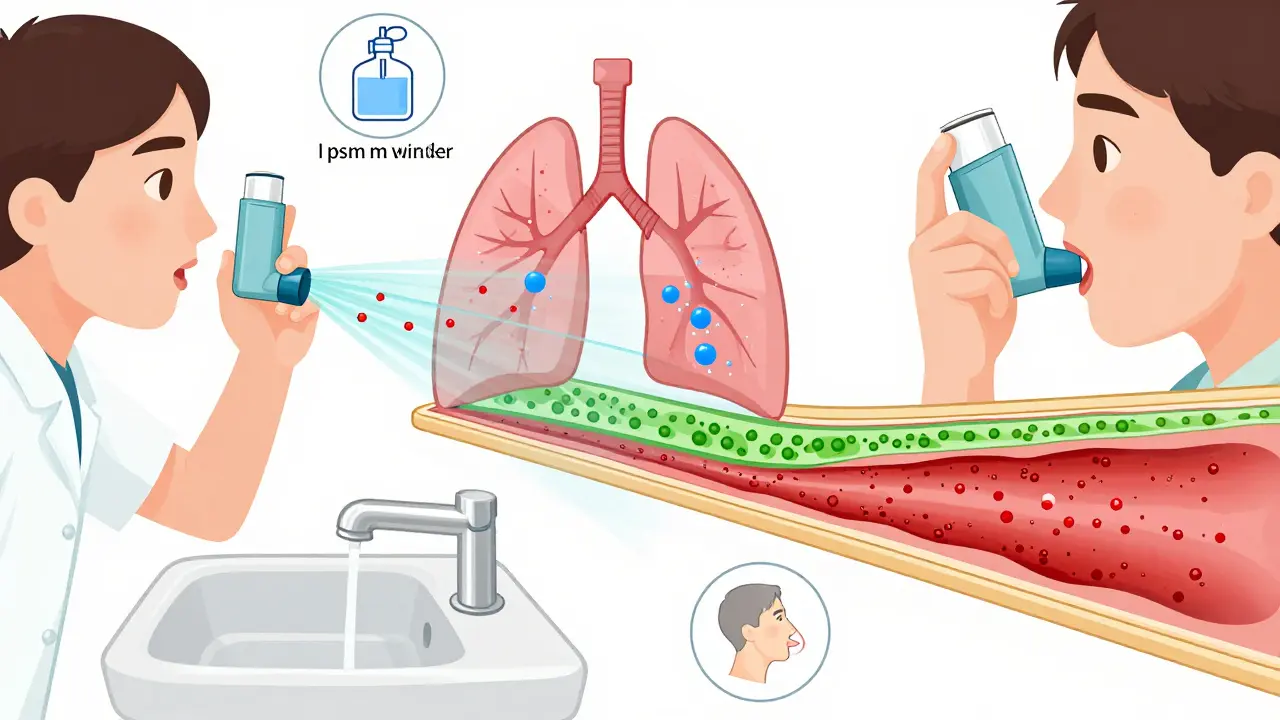
Learn how to reduce the side effects of asthma steroid inhalers with simple, proven steps. Discover which medications carry higher risks, how to spot warning signs, and what to ask your doctor to stay safe.

Track vitamin K in your diet to keep your warfarin dose stable and avoid dangerous INR swings. Learn which foods matter, how to use a food diary, and what apps work best for safer blood thinner management.
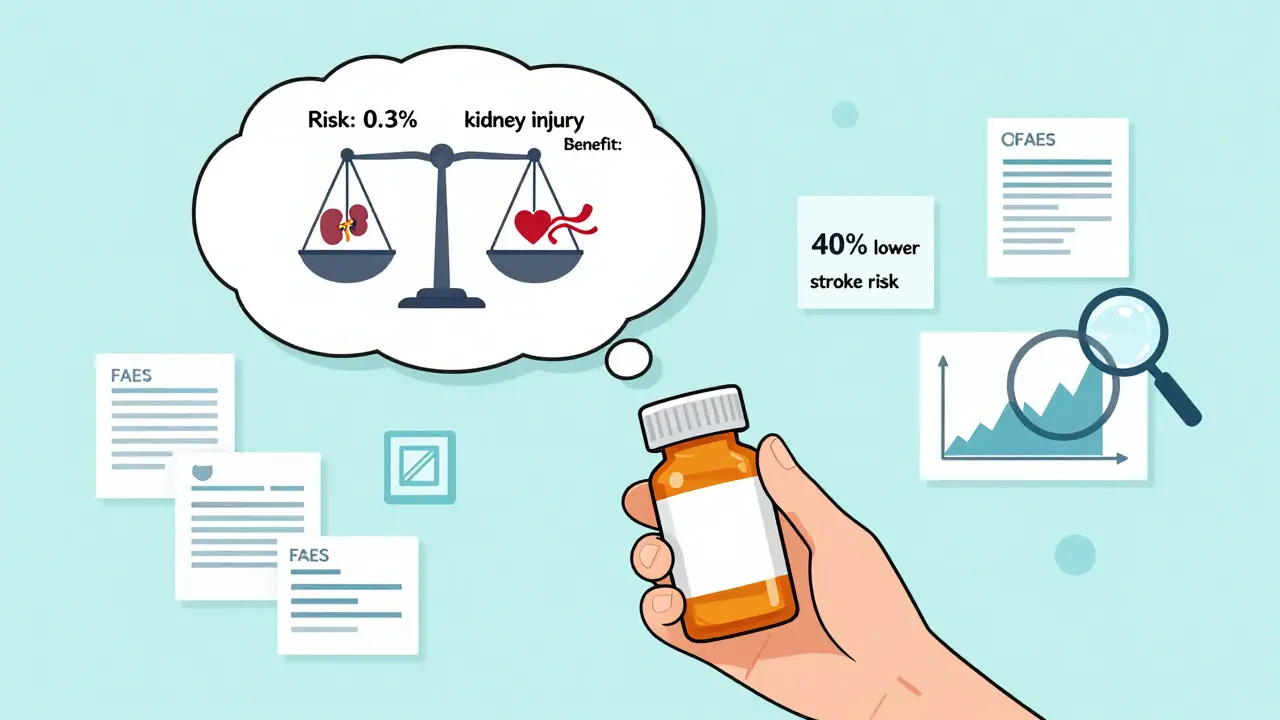
Learn how to read FDA safety announcements without panic. Understand the difference between potential signals and confirmed risks, and how to weigh the benefits of your medication against its possible side effects.
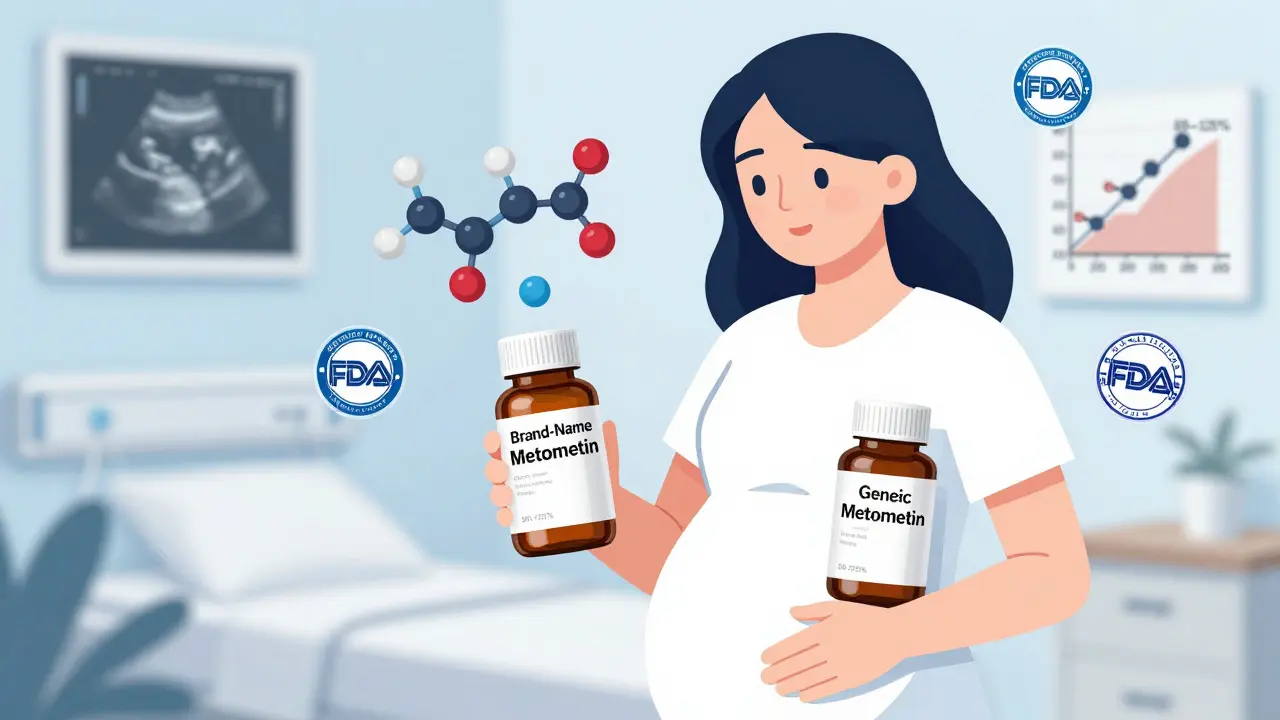
Generic medications are just as safe as brand-name drugs during pregnancy, backed by FDA standards and clinical data. Learn what the evidence says about common pregnancy meds, why concerns arise, and how to make informed choices.
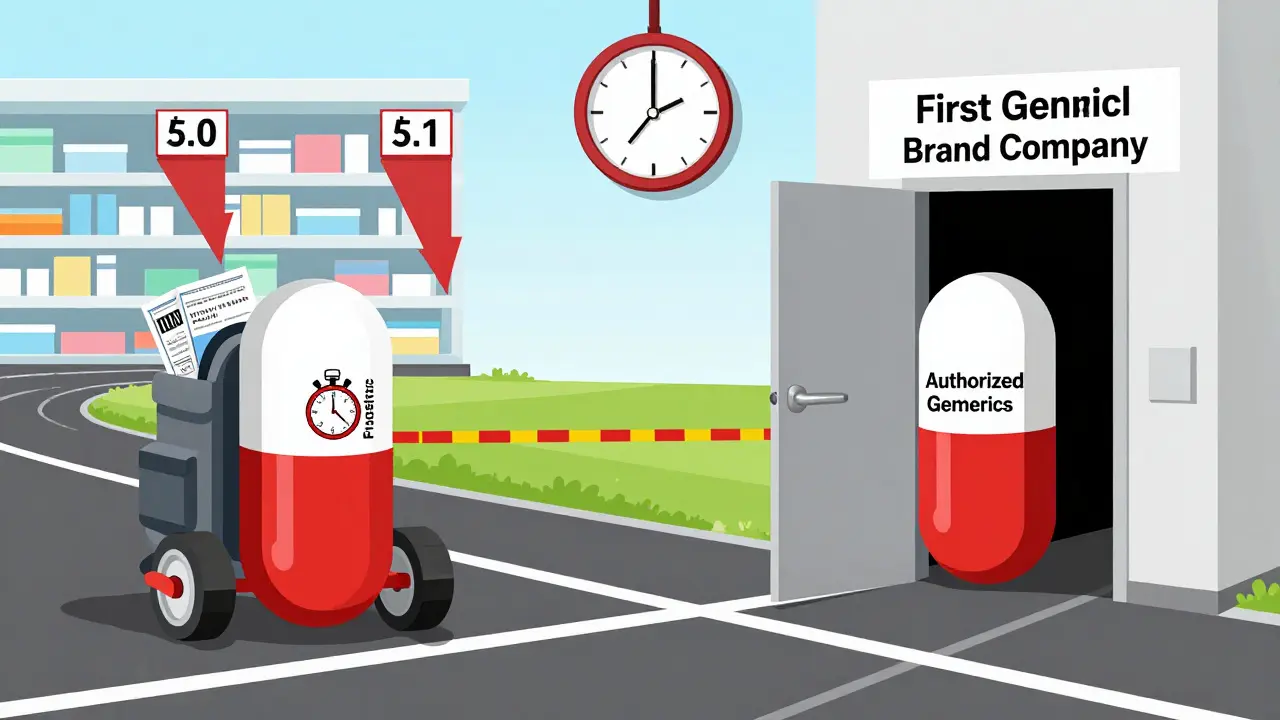
First generics and authorized generics may look the same, but their timing and origin change everything. Learn how brand companies use authorized generics to undercut independent generic manufacturers and limit price savings.
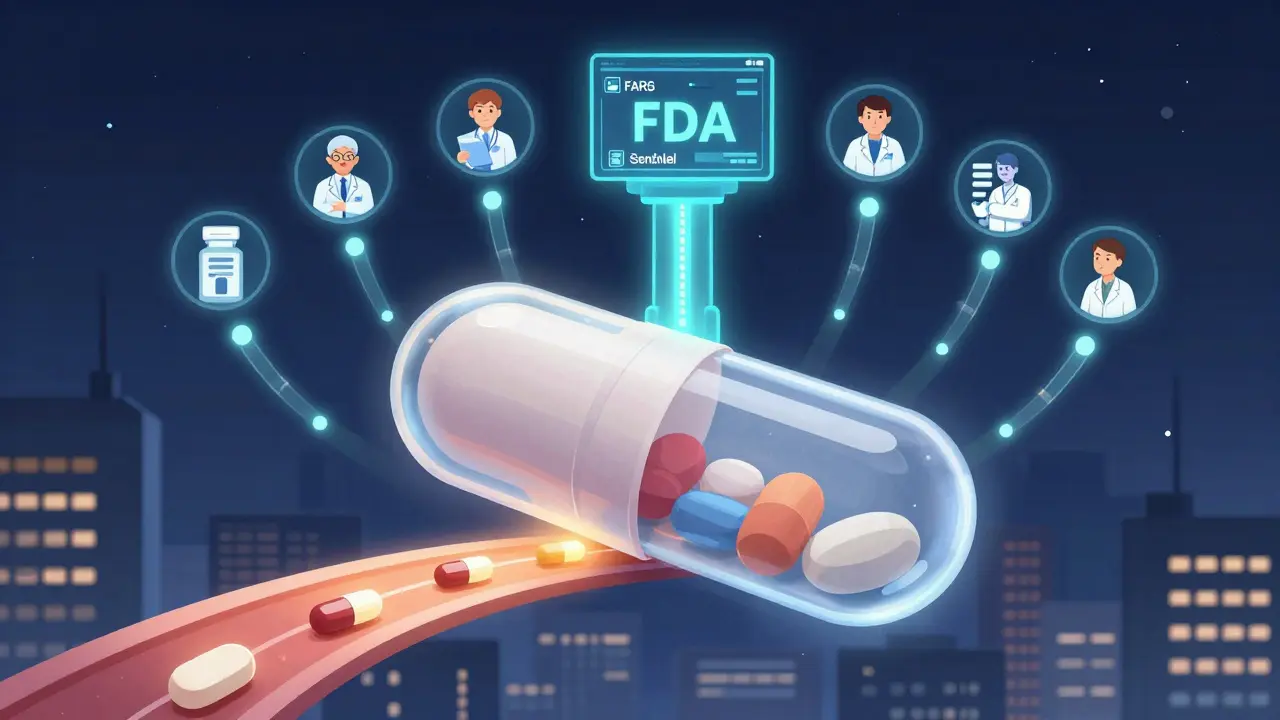
The FDA monitors generic drugs after approval using real-world data, adverse event reports, and inspections to ensure ongoing safety. Learn how systems like FAERS and Sentinel catch hidden risks that clinical trials miss.

The FDA removed the mandatory REMS program for clozapine in February 2025, but ANC monitoring remains essential. Learn how safety practices have changed and what patients and providers need to know now.

Learn the real risks of epidural and spinal procedures while on blood thinners, including timing rules, warning signs, and how to prevent life-threatening spinal hematomas.

Brand manufacturers create authorized generics-identical copies of their own drugs-to retain market share after patents expire. Learn how they're made, why they cost more than regular generics, and what this means for your prescriptions.