
Authorized generics let brand manufacturers sell their own drugs under generic labels after patent expiry. With new FDA rules and falling delays in launches, they’re becoming a faster, more transparent way to lower drug prices - not just a tactic to block competition.
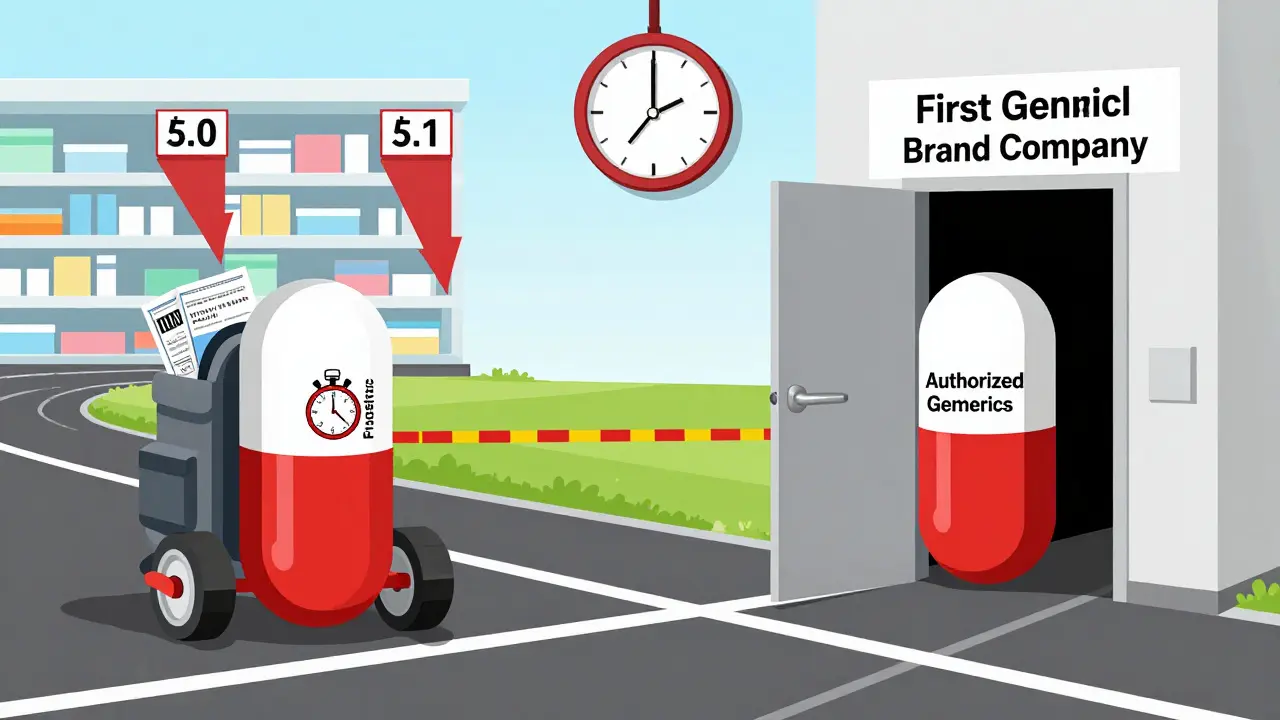
First generics and authorized generics may look the same, but their timing and origin change everything. Learn how brand companies use authorized generics to undercut independent generic manufacturers and limit price savings.

Brand manufacturers create authorized generics-identical copies of their own drugs-to retain market share after patents expire. Learn how they're made, why they cost more than regular generics, and what this means for your prescriptions.
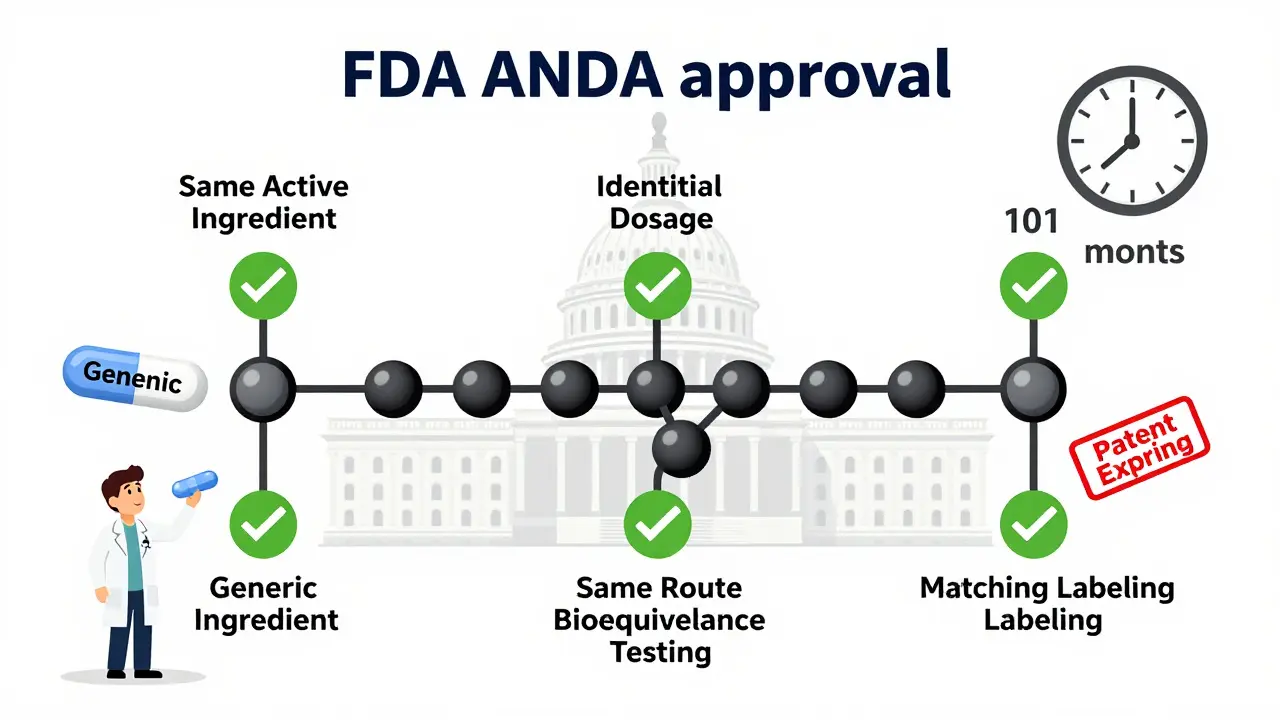
Learn the legal requirements for generic drug approval in the U.S. through the FDA's ANDA process, including bioequivalence standards, patent rules, manufacturing guidelines, and key challenges manufacturers face.