Generic and brand-name medications carry the same risk of drug interactions because they contain identical active ingredients. Scientific evidence confirms their safety and effectiveness, debunking common myths about generics.
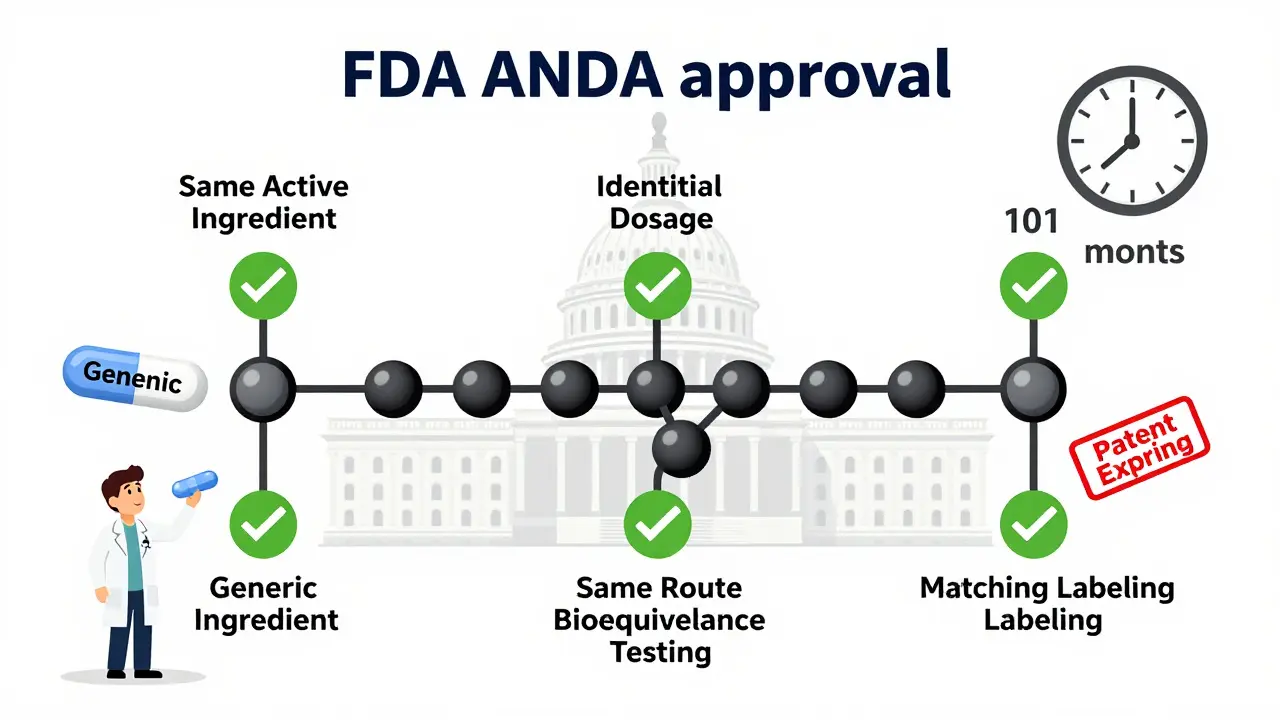
Learn the legal requirements for generic drug approval in the U.S. through the FDA's ANDA process, including bioequivalence standards, patent rules, manufacturing guidelines, and key challenges manufacturers face.
Medication reformulations change how drugs are made-not what’s in them. Learn why companies tweak formulas, how it affects your treatment, and what to do if your pill looks different.

Generic drugs work the same as brand-name medications but cost 80-85% less. Learn how they're made through reverse engineering, bioequivalence testing, and strict FDA manufacturing standards - without repeating clinical trials.