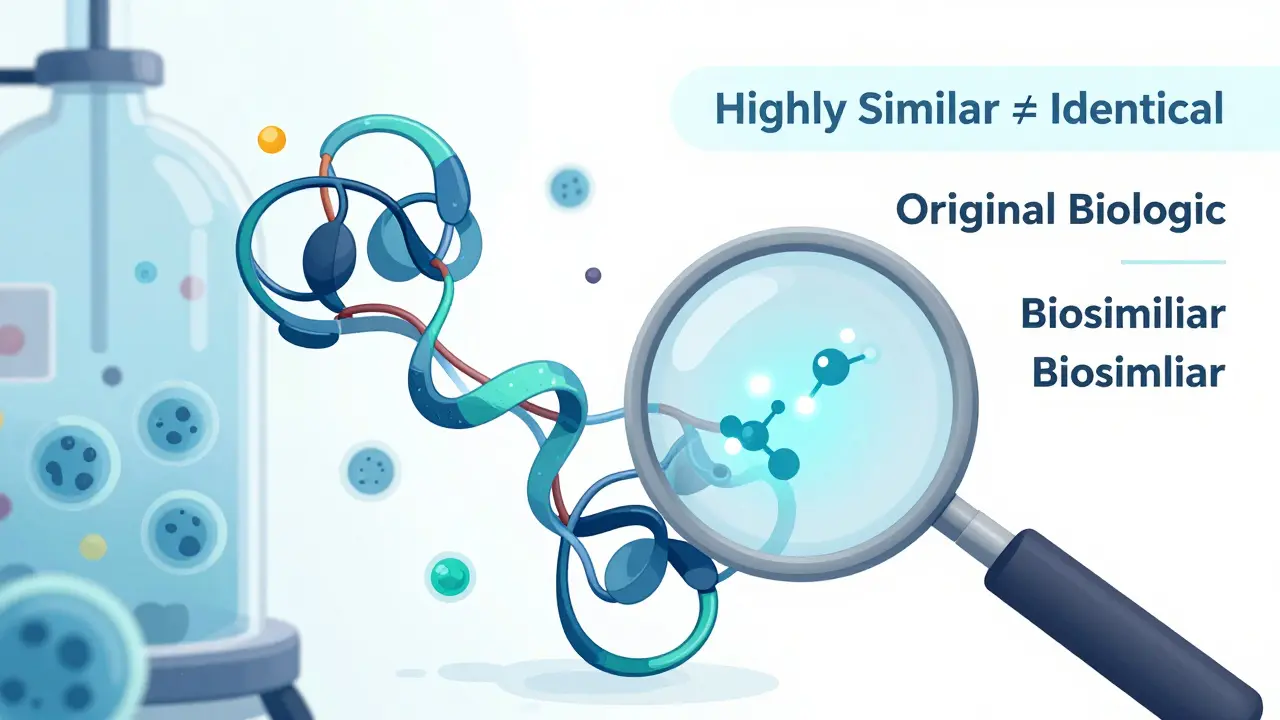
Monoclonal antibody biosimilars offer proven, cost-effective alternatives to expensive biologic drugs for cancer and autoimmune diseases. Learn which ones are approved, how they work, and why they’re changing patient care.
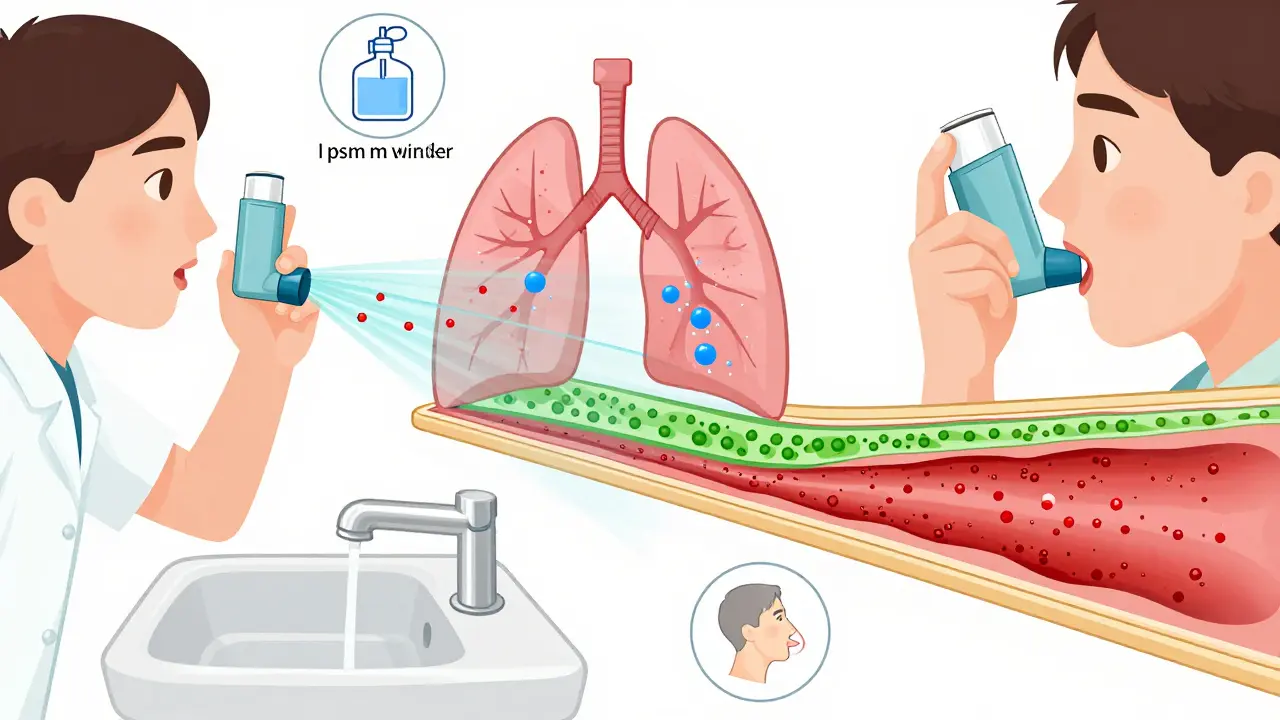
Learn how to reduce the side effects of asthma steroid inhalers with simple, proven steps. Discover which medications carry higher risks, how to spot warning signs, and what to ask your doctor to stay safe.
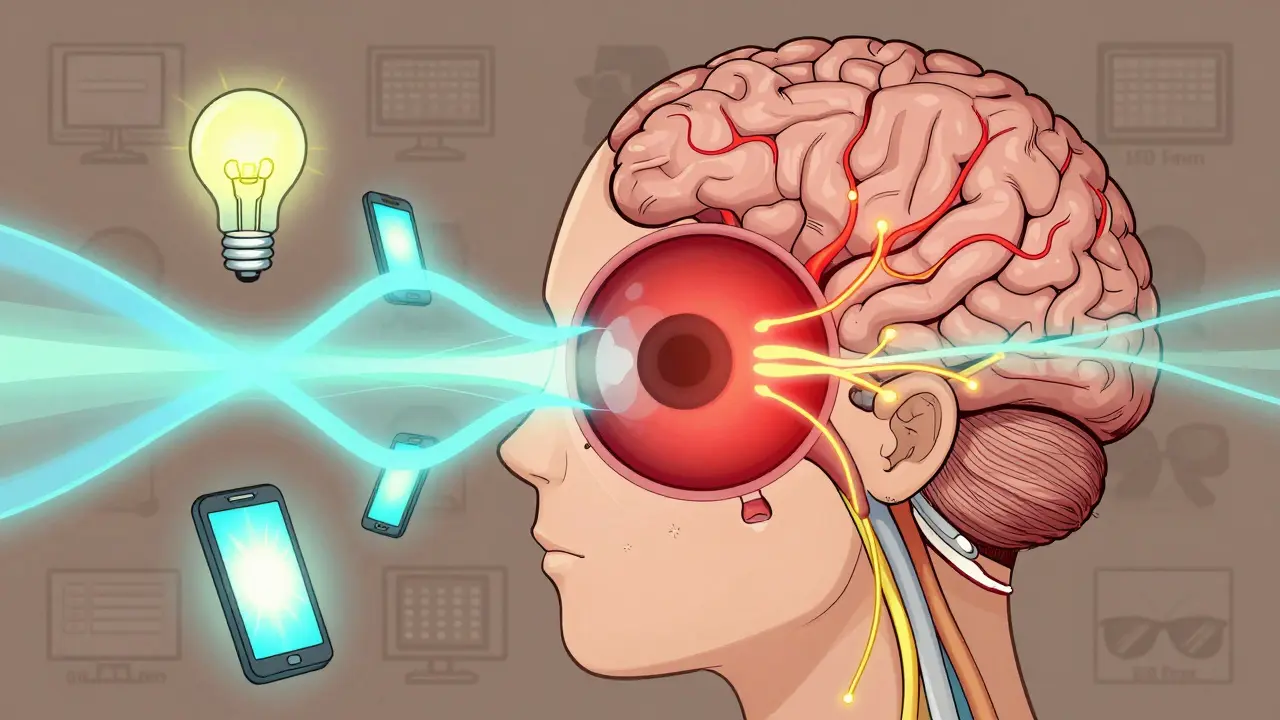
Photophobia is more than just light sensitivity-it's often a warning sign of underlying health issues. Learn the real causes, how FL-41 lenses help, and what steps to take for lasting relief.
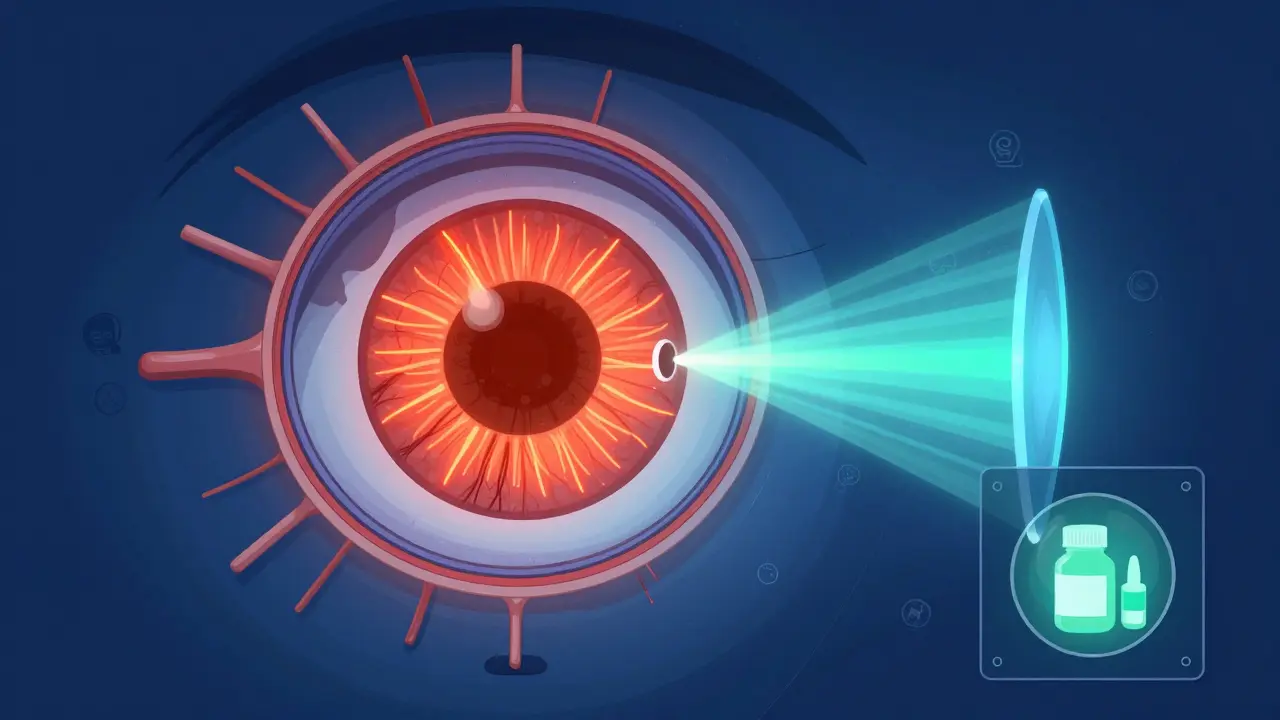
Photophobia isn't just being bothered by bright light-it's a symptom of underlying eye or neurological conditions. Learn the real causes, how FL-41 lenses help, and what treatments actually work.

Track vitamin K in your diet to keep your warfarin dose stable and avoid dangerous INR swings. Learn which foods matter, how to use a food diary, and what apps work best for safer blood thinner management.
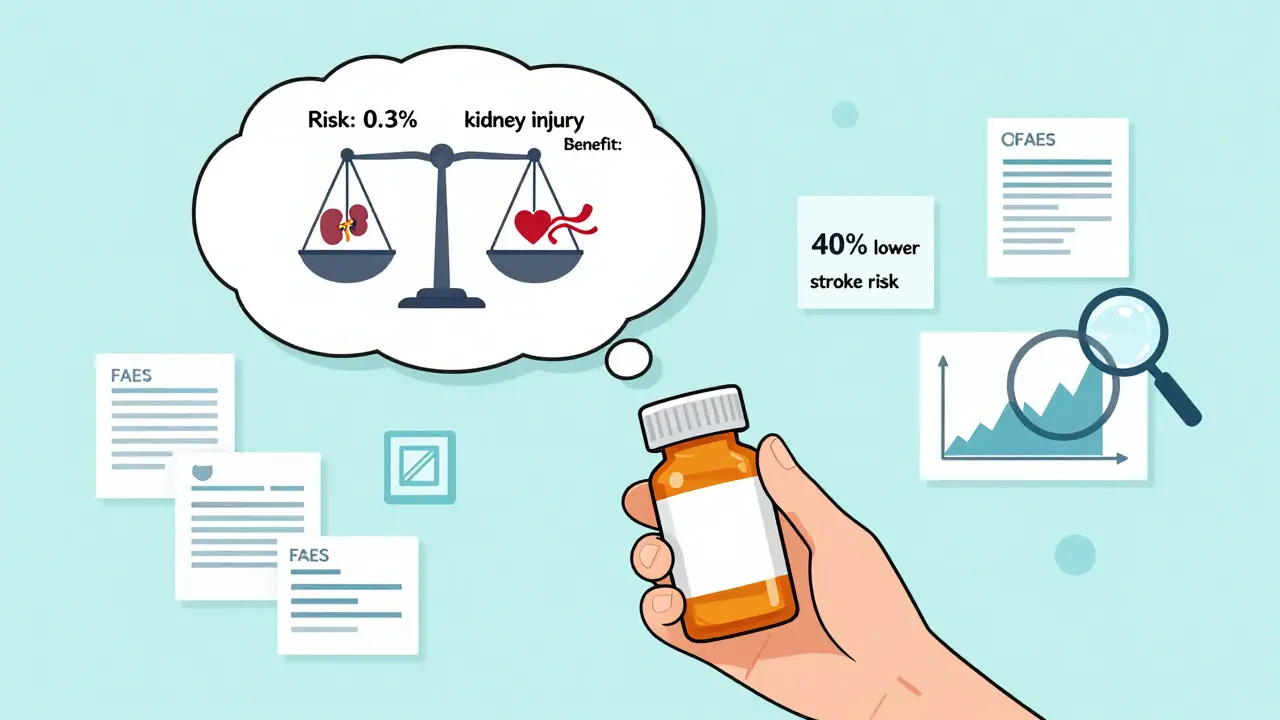
Learn how to read FDA safety announcements without panic. Understand the difference between potential signals and confirmed risks, and how to weigh the benefits of your medication against its possible side effects.
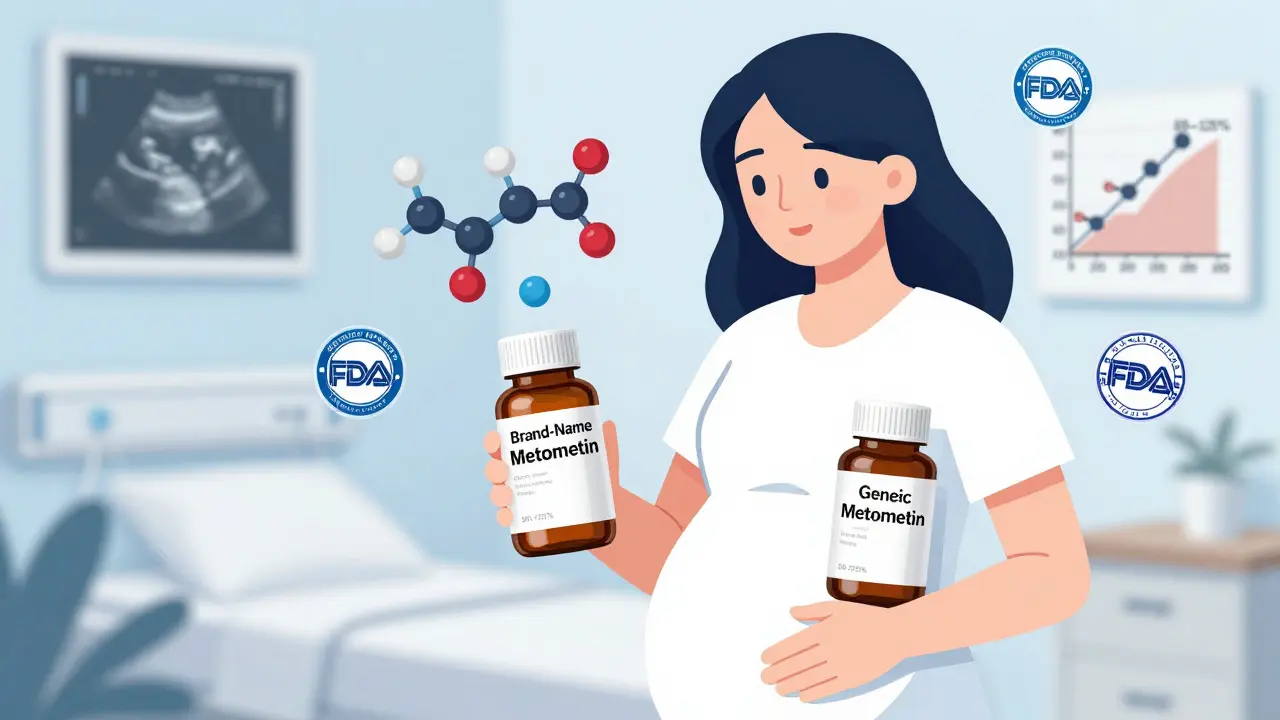
Generic medications are just as safe as brand-name drugs during pregnancy, backed by FDA standards and clinical data. Learn what the evidence says about common pregnancy meds, why concerns arise, and how to make informed choices.
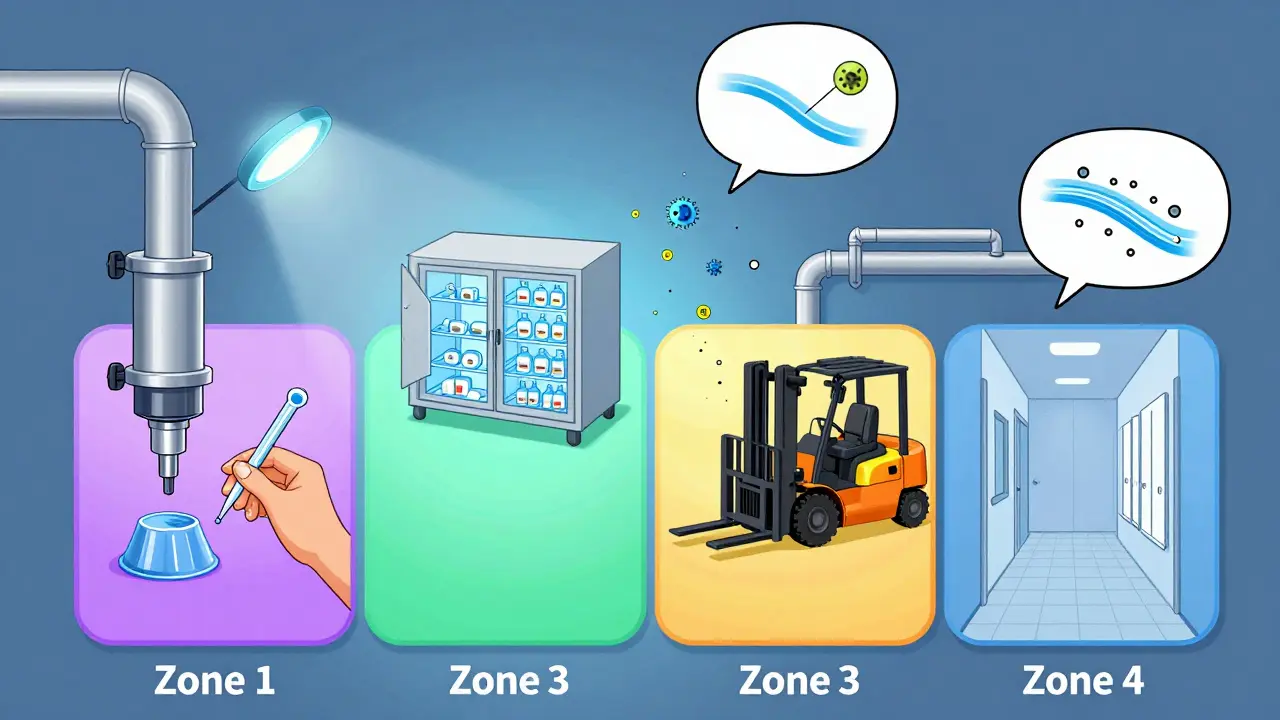
Environmental monitoring in manufacturing detects contamination before it affects products. Learn how Zone 1-4 sampling, microbial testing, and real-time data help prevent recalls and ensure safety.
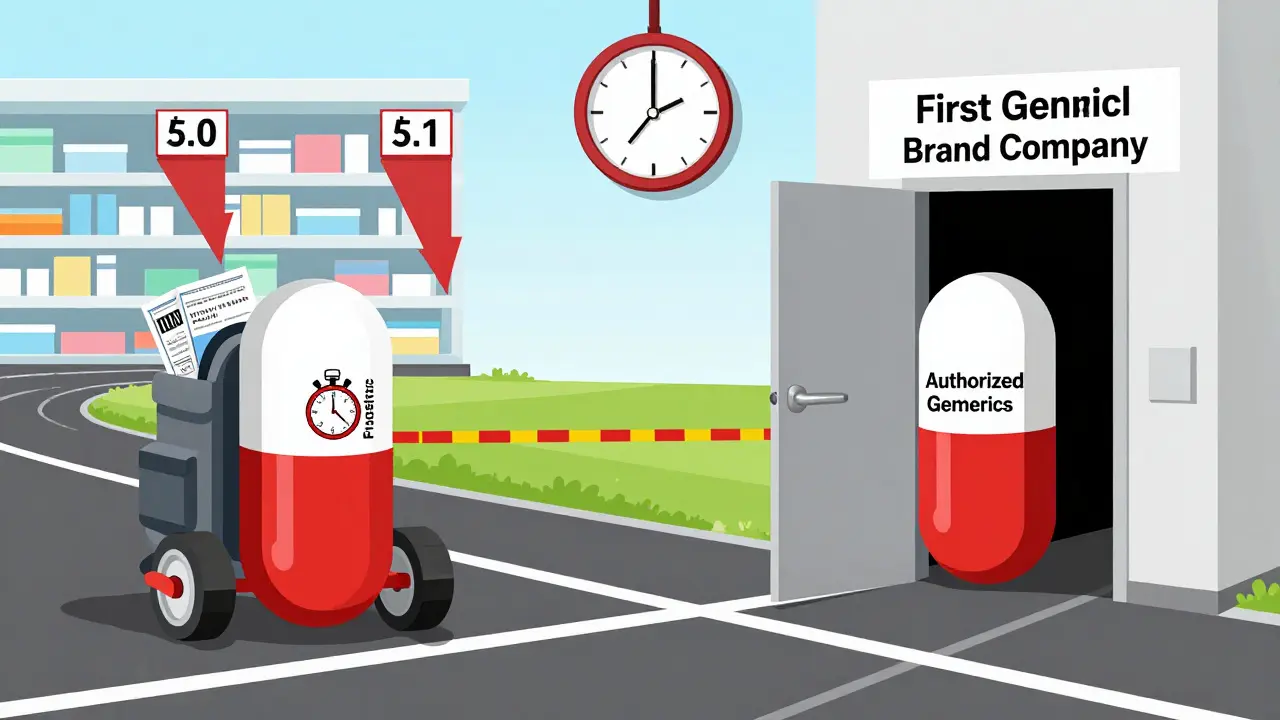
First generics and authorized generics may look the same, but their timing and origin change everything. Learn how brand companies use authorized generics to undercut independent generic manufacturers and limit price savings.
The FDA monitors generic drugs after approval using real-world data, adverse event reports, and inspections to ensure ongoing safety. Learn how systems like FAERS and Sentinel catch hidden risks that clinical trials miss.