When you pick up a generic drug, a medication that contains the same active ingredient as a brand-name drug but is sold under its chemical name. Also known as generic medication, it is approved by the FDA after proving it delivers the same therapeutic effect as the original. But approval doesn’t mean it’s always interchangeable in practice. For most people, generics work just fine—cheaper, same results. But for some, even tiny differences in how the drug is absorbed can cause serious problems.
This is where narrow therapeutic index (NTI) drugs, medications where the difference between an effective dose and a toxic one is very small. Also known as critical dose drugs, they include drugs like warfarin, levothyroxine, and certain anti-seizure medications come in. A 5% change in blood levels might mean the drug stops working—or causes poisoning. That’s why therapeutic drug monitoring, the process of measuring drug levels in the blood to ensure they stay within a safe and effective range is essential for patients on these drugs. The FDA says generics are bioequivalent, but real-world data shows that switching between different generic brands—even for the same drug—can lead to hospitalizations in vulnerable patients.
Why does this happen? Generic drugs can use different fillers, coatings, or manufacturing processes. These don’t affect the active ingredient, but they can change how fast or how well the body absorbs it. For a blood pressure pill like lisinopril, that’s usually fine. For a drug like phenytoin used to control seizures, it’s not. One patient might switch from one generic to another and suddenly have seizures. Another might start bleeding uncontrollably after a generic warfarin substitution. These aren’t rare cases—they’re documented in medical journals and emergency rooms across the country.
The system assumes all generics are equal, but your body doesn’t always agree. That’s why doctors who treat complex conditions—like Parkinson’s, epilepsy, or heart failure—often prefer to stick with one brand or generic version. They know switching can break the balance their patient has worked hard to achieve. And if you’re on one of these high-risk drugs, you should know your prescription isn’t just a label—it’s a treatment plan. If your pharmacy switches your pill without telling you, ask. If you feel different after a refill change, tell your doctor. You’re not being difficult—you’re being smart.
What you’ll find below are real stories and clear breakdowns of how generic drug approval plays out in actual medical practice. From patients who had to fight to stay on the same version of their medication, to doctors who use therapeutic drug monitoring to catch problems before they turn dangerous. You’ll see how NTI drugs behave differently from regular generics, why some substitutions are safe and others aren’t, and what you can do to protect yourself. This isn’t about theory. It’s about what happens when a pill changes, and your body notices.
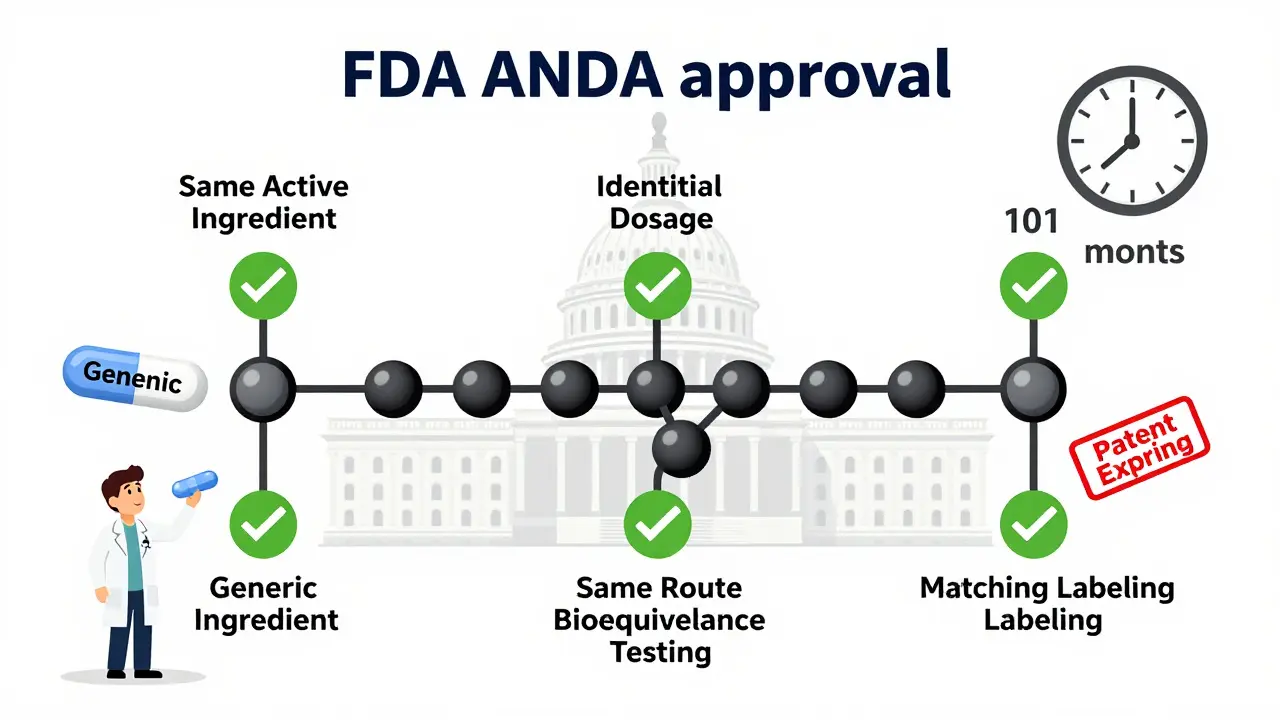
Learn the legal requirements for generic drug approval in the U.S. through the FDA's ANDA process, including bioequivalence standards, patent rules, manufacturing guidelines, and key challenges manufacturers face.

Bioequivalence studies prove generic drugs work the same as brand-name versions by measuring how quickly and how much of the drug enters the bloodstream. This step-by-step process ensures safety, effectiveness, and cost savings.