When working with clinical trial, a systematic study that tests how well a medical intervention works in people. Also known as medical study, a drug, any substance used for treatment, prevention, or diagnosis is usually the focus of the investigation. To separate true effects from chance, researchers often give one group a placebo, an inactive substance that looks like the active medication. The gold‑standard design is the randomized controlled trial, where participants are randomly assigned to receive either the intervention or the placebo, minimizing bias. These building blocks let scientists answer if a new therapy is safe, effective, and worth moving forward.
Every clinical trial moves through distinct phases. Phase I checks safety in a small group of healthy volunteers. Phase II expands to patients to gauge efficacy and optimal dosing. Phase III involves hundreds to thousands, confirming benefits and monitoring side effects across diverse populations. Finally, Phase IV watches the drug after market approval, spotting rare problems and long‑term outcomes. Throughout, patient recruitment is a make‑or‑break factor; enrolling the right mix of ages, genders, and health statuses ensures results apply to the real world. Regulators like the FDA evaluate the trial data before granting approval, and ethics committees safeguard participant rights at every step.
Because trials generate massive data, the way researchers interpret results matters. Statistical significance tells whether an observed effect is likely due to the intervention rather than chance, while clinical relevance asks if the benefit matters to patients. Endpoints—such as reduced heart attacks, lower blood pressure, or improved quality of life—are chosen before the study starts to avoid cherry‑picking outcomes. When a trial shows a clear advantage, the findings often filter into drug comparison guides, dosage recommendations, and safety warnings that healthcare providers rely on daily.
Below you’ll find a curated collection of medication reviews, dosage tips, and safety overviews—all grounded in the evidence that comes out of well‑designed clinical trials. Dive in to see how research translates into the practical advice you need for everyday health decisions.
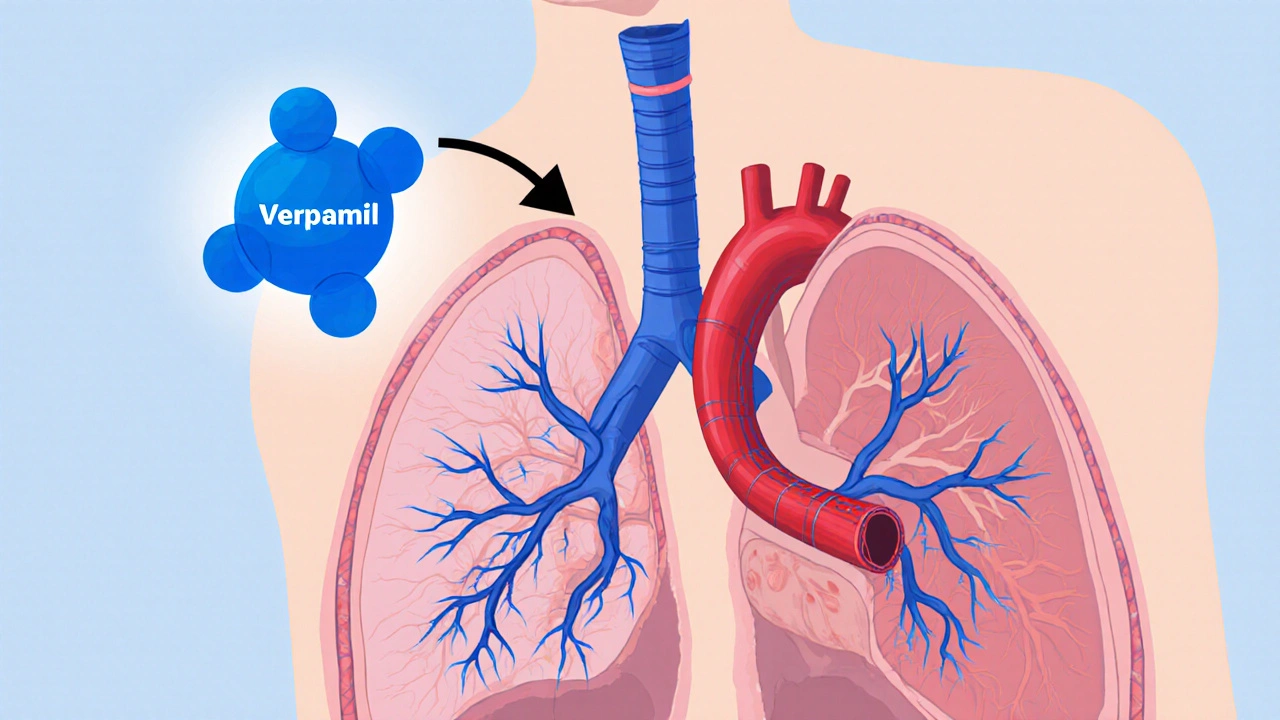
Explore how verapamil works for idiopathic pulmonary arterial hypertension, the evidence behind its use, patient selection, dosing, and future research.