When you pick up a generic pill at the pharmacy, you expect it to work just like the brand-name version. And for the most part, it does. The FDA requires generics to have the same active ingredients, strength, and dosage form. But here’s something most people don’t realize: the safety warning on that generic bottle might be outdated.
How Generic Drug Warnings Work (And Why They’re Often Behind)
Generic drug makers can’t change their warning labels on their own. Even if new safety data shows a risk - like liver damage, dangerous interactions, or rare side effects - they must wait for the original brand-name company to update its label first. Then, and only then, can the generic manufacturer follow suit. This rule comes from the Hatch-Waxman Act of 1984, designed to speed up generic approvals and lower drug costs. But it’s created a dangerous blind spot.Let’s say a brand-name drug’s label gets updated to warn about a new heart rhythm risk. That update goes out to doctors, pharmacists, and patients. But if the generic version still carries the old label, patients taking the cheaper version might not know. And since over 90% of prescriptions in the U.S. are filled with generics, that’s millions of people potentially missing critical safety info.
The MedWatch System and What It Can’t Do
The FDA tracks drug safety through its MedWatch program. Anyone - doctors, pharmacists, or even patients - can report side effects. These reports go into the FDA Adverse Event Reporting System (FAERS), which is monitored monthly. For example, when the first generic version of Rexulti hit the market in 2019, the FDA watched closely for any safety signals. None were found.But here’s the catch: MedWatch collects reports. It doesn’t force label changes for generics. Only the brand-name company can trigger a label update using a process called Changes Being Effected (CBE-0). This lets them add new warnings without waiting for FDA approval. Generic manufacturers can’t use this shortcut. They’re stuck waiting - sometimes for months or even over a year - while patients keep taking the drug with outdated warnings.
Why This Matters: Real Risks, Real Patients
Differences in inactive ingredients - like dyes, fillers, or preservatives - can affect how a drug behaves in the body. The FDA allows some variation in these excipients for generics, except in special cases like eye drops or IV meds. But even small changes can cause problems for sensitive patients. One person might tolerate a generic version fine. Another, with allergies or liver issues, might react badly - and not know why, because the label doesn’t warn them.Imagine someone with kidney disease taking a generic blood pressure pill. The brand-name label clearly says to avoid it if creatinine levels are high. But the generic label doesn’t mention it. The patient’s doctor doesn’t know the difference. The pharmacist just fills the prescription. The patient ends up in the hospital. This isn’t hypothetical. It’s happened. And it’s preventable.
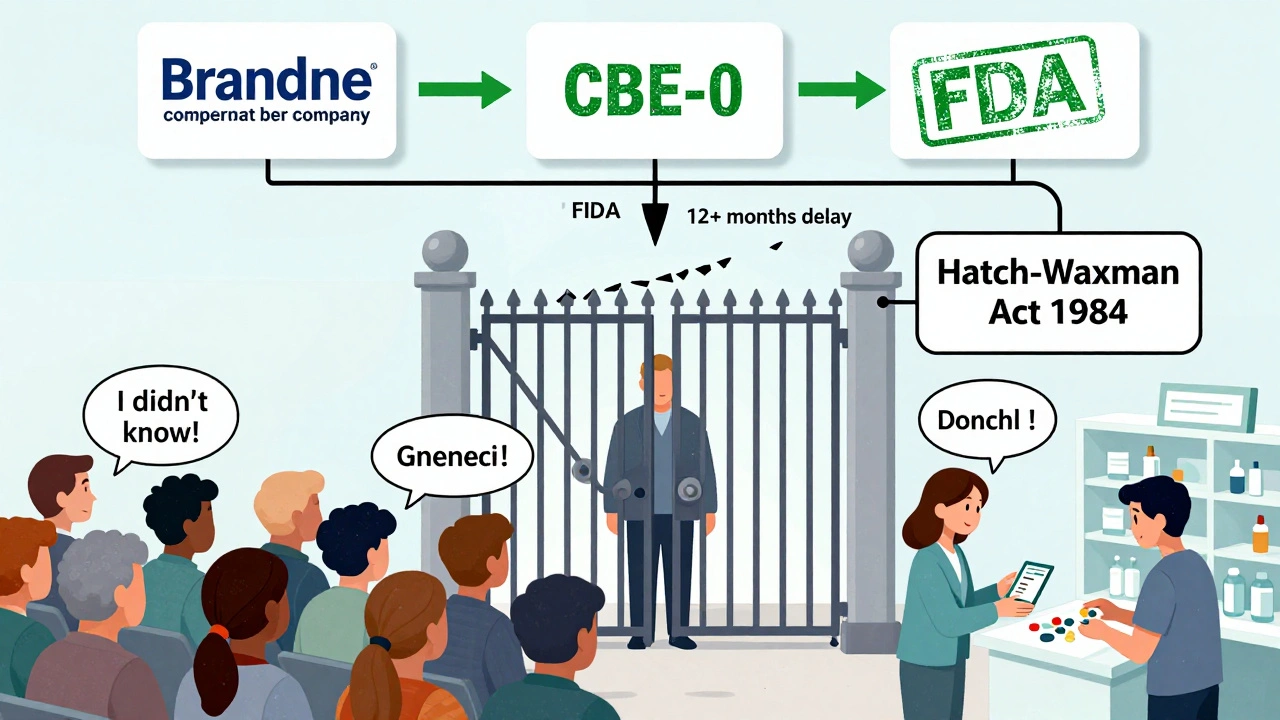
The FDA’s Proposed Fix - And Why It’s Stuck
Back in 2013, the FDA proposed letting generic manufacturers use the CBE-0 process too. That would let them update warnings independently when new safety data emerges. Over 27 consumer health groups, including the American Association for Justice, backed the change. Their argument? If insurance companies force you to take a generic, you deserve the same safety info as someone taking the brand-name drug.The generic drug industry pushed back. They argued that giving them the power to change labels could open them up to lawsuits. If they add a warning and the FDA later says it wasn’t needed, they could be sued for scaring patients. If they don’t add it and someone gets hurt, they could be sued for not warning. Either way, they lose.
And legally, it’s messy. Brand-name companies have already been sued for failing to update warnings quickly enough. If generics get the same responsibility, who’s liable when something goes wrong? The courts haven’t answered that yet.
What’s Being Done Right Now?
The FDA still hasn’t made a final decision. As of late 2024, the 2013 proposal remains under review. But they’re not sitting idle. The Office of Generic Drugs now does proactive monitoring. When a new generic hits the market, especially a complex one like a patch or extended-release capsule, they check if the look, size, or shape might confuse patients. They’ve even looked into whether different colors or markings could cause dosing errors.The FDA also runs public education campaigns. Their website has pages like “Generic Drugs: Questions & Answers” and “Generic Drug Facts” to help people understand what’s the same - and what might be different - between brand and generic versions. They post safety alerts on their Drug Safety and Availability page, including recent actions like the 2024 withdrawal of Oxbryta due to safety concerns.
What You Can Do as a Patient
You can’t control the label. But you can protect yourself.- Ask your pharmacist: “Is this generic the same as the brand in terms of warnings?” They might not know, but it’s worth asking.
- Check the FDA’s website for recent safety alerts. Search “FDA drug safety alerts” and look for your medication by name.
- If you’re on a generic drug and experience a new side effect, report it to MedWatch. Even one report can help trigger a review.
- Keep a list of all your medications - brand and generic - and bring it to every doctor visit. If you switch from brand to generic, tell your doctor.
- Don’t assume “same drug = same risks.” The active ingredient is the same. The warning label might not be.
The Bigger Picture: Cost vs. Safety
Generics save the U.S. healthcare system an estimated $300 billion every year. That’s huge. But safety shouldn’t be the price we pay for savings. The system was built to make drugs cheaper, not to make them less safe. Yet today, the rules are still stuck in 1984 - while medicine has moved far beyond it.Complex generics - like inhalers, injectables, and topical creams - are growing fast. These aren’t simple pills. They’re engineered products. A tiny difference in how the drug is released can change how it works in your body. And right now, the safety labels for these complex generics still follow the same outdated rules.
Until the FDA acts, patients are left in the middle. You’re getting a drug that works. But you might not be getting the full story about what it can do - or harm.
What’s Next?
The pressure is building. Consumer groups aren’t giving up. Legal experts are watching for court cases that could force a change. And with more complex generics hitting the market, the FDA can’t ignore the issue forever.For now, the best defense is awareness. Know your meds. Know your warnings. And don’t assume cheap means the same.
Can generic drugs have different side effects than brand-name drugs?
The active ingredient is identical, so the core side effects are the same. But differences in inactive ingredients - like dyes, fillers, or preservatives - can cause reactions in sensitive individuals. Some people report different tolerability, like stomach upset or headaches, when switching to a generic. These aren’t always listed on the label, because the FDA doesn’t require generics to match excipients exactly - except for certain types like eye drops or IVs.
Why can’t generic manufacturers update their own safety labels?
Under the Hatch-Waxman Act of 1984, generic drug makers must mirror the label of the original brand-name drug. They can’t add new warnings unless the brand-name company updates theirs first. This rule was meant to speed up generic approvals but has created a lag in safety communications. The FDA proposed changing this in 2013, but the rule hasn’t been finalized.
How do I know if my generic drug’s warning label is up to date?
Check the FDA’s Drug Safety and Availability page or search for your drug’s name + “FDA safety alert.” You can also compare the prescribing information on the brand-name manufacturer’s website with the package insert that came with your generic. If the brand has a new warning and your generic doesn’t, it’s likely outdated. Contact your pharmacist or doctor to confirm.
Are complex generics more dangerous because of outdated labels?
They can be. Complex generics - like patches, inhalers, or extended-release injections - rely on precise delivery systems. Even small differences in how the drug is released can affect safety. If the label doesn’t reflect new data on these delivery systems, patients may be at higher risk. The FDA is now doing more proactive reviews for these types of drugs, but the labeling rules still haven’t changed.
Should I avoid generic drugs because of safety concerns?
No. The vast majority of generic drugs are safe and effective. The FDA approves them using the same strict standards as brand-name drugs. The issue isn’t the drugs themselves - it’s the outdated safety labels. Stay informed. Report side effects. Ask questions. Don’t skip your meds, but don’t assume you have all the facts.

Tiffany Sowby
December 9, 2025 AT 07:33This is why I stopped trusting generics entirely. I took one for my anxiety and ended up in the ER thinking I was having a heart attack. Turns out the label didn't mention the interaction with my coffee. My brand-name prescription? Clear as day. Now I pay out of pocket just so I don't die from a typo.
Asset Finance Komrade
December 10, 2025 AT 18:54One must contemplate the epistemological paradox inherent in pharmaceutical regulation: if safety data is emergent, and liability is deferred, then the very notion of informed consent becomes a performative illusion. The Hatch-Waxman Act, a relic of Reagan-era pragmatism, now functions as a legal scaffold for systemic negligence. We have commodified health, and in doing so, rendered the patient a passive node in a supply chain.
Jennifer Blandford
December 12, 2025 AT 17:38I just want to say thank you for writing this. I’ve been terrified to speak up about this, but my mom had a stroke last year after switching to a generic blood thinner. The label didn’t say anything about her kidney condition. We found out later the brand had updated the warning 8 months before. She’s okay now, but it broke my heart. Please keep pushing for change 💔
Ronald Ezamaru
December 13, 2025 AT 18:20There’s a real gap here between regulatory logic and patient reality. The FDA’s approval process for generics is rigorous on bioequivalence, but the labeling lag is a blind spot. Pharmacists aren’t trained to compare label versions across brands and generics. Patients assume ‘same drug’ means ‘same risks.’ It’s not malice-it’s systemic inertia. The 2013 proposal was sound. If the FDA can’t act now, Congress should step in.
Iris Carmen
December 14, 2025 AT 19:31so i switched to generic adderall last year and my brain felt like mush for 3 weeks. i thought i was just depressed but turns out the filler was messing with my gut and my meds werent absorbing. i went back to brand and boom. back to normal. no one told me this could happen. why do they even let these things out if they cant even match the warnings?
Kathy Haverly
December 14, 2025 AT 22:10Of course the FDA won’t fix this. They’re owned by Big Pharma. The brand-name companies profit from the delay-they get to keep charging premium prices while generics sit on outdated labels. This isn’t negligence. It’s a business model. And you think your insurance company cares? They push generics because it’s cheaper, not because it’s safer. Wake up.
Andrea Petrov
December 16, 2025 AT 01:29Have you ever noticed that every time a new safety alert comes out, it’s always for a generic drug that’s been on the market for 10+ years? Coincidence? Or is this just how they test the population? I’ve seen reports of people getting rashes from generic omeprazole. The brand never had that. I think they’re using us as guinea pigs. The FDA knows. They just don’t care.
Suzanne Johnston
December 16, 2025 AT 06:23This is exactly why we need a patient-centered regulatory framework. The current system treats drug safety like a legal formality, not a human right. If a patient is forced by their insurer to take a generic, they deserve the same transparency as someone taking the brand. The FDA’s delay isn’t bureaucratic-it’s moral. We can fix this. We just need the will.
Graham Abbas
December 17, 2025 AT 23:48I work in pharmacy. I see this every day. We fill generics because that’s what’s on the script. We don’t have time to cross-check label versions with the FDA’s website. And even if we did, most patients wouldn’t understand the difference. We’re stuck in the middle. Someone needs to give us a tool-like a real-time label sync system. Until then, we’re just guessing.
Haley P Law
December 19, 2025 AT 03:09OMG I just realized my thyroid med is generic and the label doesn’t say anything about dairy interactions. I drink almond milk every morning. I’m gonna die. I’m gonna die. I’m gonna die. 😭💀 I’m calling my doctor right now. This is a nightmare. Someone please tell me I’m not the only one who didn’t know this.
Andrea DeWinter
December 19, 2025 AT 06:54Don’t panic but do check your labels. If you’re on a generic and you’ve had a weird side effect you can’t explain, report it to MedWatch. One report might not do much but 500 could trigger a review. And if your doctor doesn’t know the difference between brand and generic warnings, ask them to look it up. It’s not their fault they don’t know-no one taught them. But you can help change that