When you buy a bottle of medicine, a ready-to-eat meal, or a jar of lotion, you expect it to be safe. But what happens behind the scenes to make sure it is? In manufacturing facilities-from pharmaceutical plants to food processing lines-environmental monitoring is the invisible shield that stops contamination before it reaches you. It’s not just about cleaning surfaces. It’s about knowing exactly where germs, chemicals, or particles are hiding, and catching them before they ruin a batch or worse, make someone sick.
Why Environmental Monitoring Isn’t Optional
In 2022, foodborne illnesses tied to environmental contamination cost the U.S. economy over $77 billion. That’s not a typo. It’s billions lost to recalls, lawsuits, and lost trust. The FDA, CDC, and EU regulators don’t treat this as a suggestion. They treat it as a requirement. If your facility makes anything that touches people-food, drugs, cosmetics-you’re legally obligated to monitor your environment. Environmental monitoring isn’t about reacting to outbreaks. It’s about preventing them. Think of it like a smoke detector in your home. You don’t wait for the fire to start before installing one. You install it because you know fires can happen. Same logic applies in a production facility. A single Listeria cell on a conveyor belt can contaminate thousands of products. Monitoring catches those risks before they escalate.The Zone System: How to Map Your Risk
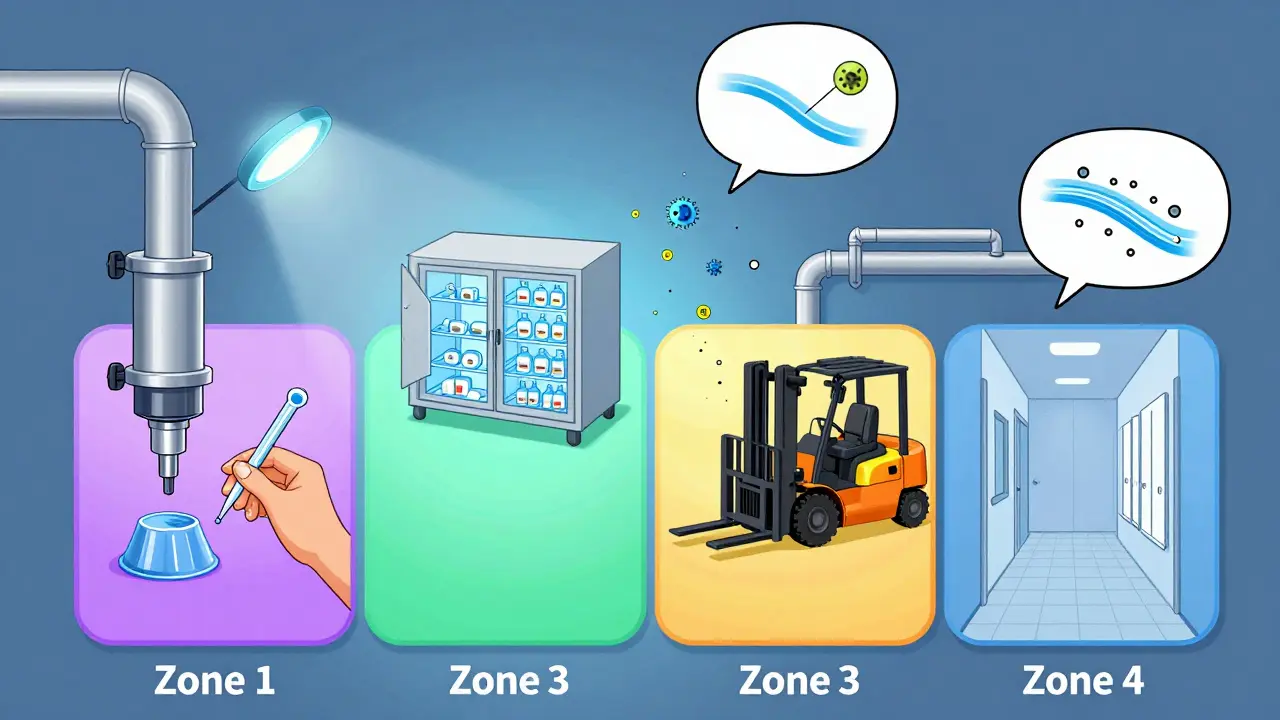
Every facility, no matter the industry, uses the same basic framework: the Zone System. It divides your facility into four risk levels based on how close a surface is to the product.- Zone 1: Direct product contact surfaces-slicers, mixers, filling nozzles, packaging rollers. These are the highest risk. If something grows here, it goes straight into your product.
- Zone 2: Surfaces near product contact areas-equipment housings, refrigeration units, nearby walls. Contamination here can easily spread to Zone 1.
- Zone 3: Remote but still in production areas-forklifts, storage carts, overhead pipes. These are often ignored, but they’re the source of 62% of contamination events in labs, according to PPD Laboratories.
- Zone 4: Outside production-hallways, restrooms, break rooms. Low risk, but still monitored for trends.
What You Test For-and How
You don’t test for everything. You test for what matters based on your product and process.- Microbes: Listeria monocytogenes and Salmonella are the top targets in food. In pharma, it’s bacteria like Pseudomonas and fungi like Aspergillus. Swabs and sponges collect samples from surfaces. Air samplers pull in cubic meters of air to count colony-forming units (CFU/m³).
- Particulates: In cleanrooms, even invisible dust can ruin a drug. ISO Class 5 (EU Grade B) cleanrooms require continuous particle counting.
- Water quality: Pharmaceutical facilities test purified water for total organic carbon (TOC) and conductivity to meet USP <645> standards. Food plants check municipal water against EPA rules.
- Chemicals and metals: Inductively Coupled Plasma (ICP) detects trace metals. Chromatography (HPLC, GC) finds pesticide residues or cleaning solvent leftovers.
How Often Should You Sample?
Frequency isn’t random. It’s risk-based.- Zone 1: Daily to weekly. RTE food plants must test for Listeria weekly. Pharmacies test after every cleaning cycle.
- Zone 2: Weekly to monthly. If a Zone 1 surface is cleaned daily, Zone 2 might be checked every 3-5 days.
- Zone 3: Monthly. Even if it’s ‘low risk,’ you still need data. Sporadic contamination often starts here.
- Zone 4: Quarterly. Mostly for trend analysis. A sudden spike in Zone 4 could mean a ventilation issue or pest problem.
Common Mistakes That Cost Companies Millions
Even the best-intentioned programs fail because of simple oversights.- Unclean samplers: The CDC says 68% of facilities don’t sterilize air samplers properly. A dirty sampler introduces its own contamination.
- Fragmented data: ATP results, microbial tests, allergen checks, and chemical tests are often tracked in separate spreadsheets. No one connects the dots. That’s why 37% of facilities struggle with data integration.
- Undertrained staff: The FDA recommends 40 hours of hands-on training before someone can collect samples. Many facilities train staff for 2 hours and send them out. Results? Inconsistent technique, missed zones, false negatives.
- Ignoring trends: One positive result? Maybe a fluke. Three positives in the same spot over three months? That’s a pattern. Most facilities don’t analyze trends-they just log results and move on.

The Future: AI, Real-Time Data, and Faster Results
The industry is changing fast. The FDA’s 2023 draft guidance pushes for rapid methods like next-generation sequencing (NGS), which can identify pathogens in under 24 hours instead of 72. AI-powered analytics are starting to predict contamination risks based on humidity, temperature, foot traffic, and past test results. By 2027, 38% of environmental monitoring systems are expected to use AI. That’s up from just 12% in 2022. Real-time data dashboards are replacing paper logs. If a Zone 1 surface shows a spike in ATP readings, the system can flag it immediately-and even pause production until it’s cleared. Regulations are catching up. EU Annex 1 now requires continuous monitoring of critical parameters. That means sensors on air filters, humidity controls, and water lines must stream data 24/7. Facilities that haven’t upgraded are already falling out of compliance.What You Need to Start (or Improve) Your Program
If you’re setting up your first program-or trying to fix a broken one-here’s what works:- Map your zones. Don’t guess. Walk the floor. Trace how product moves. Identify every surface that could touch it or get near it.
- Define your analytes. What’s your biggest threat? Listeria? Mold? Metal residue? Test for that first.
- Train your team. 40 hours isn’t optional. Use certified trainers. Film procedures. Test them.
- Integrate your data. Use one platform to track ATP, microbial, and chemical results. Look for patterns.
- Review monthly. Don’t wait for an audit. Check your own data. Are you getting the same bugs in the same spot? Time to re-clean or re-design.
Final Thought: It’s Not About Compliance. It’s About Control.
Environmental monitoring isn’t a box to tick for regulators. It’s a tool to control your environment. The most successful facilities don’t just test-they act on what they find. They fix leaks. They retrain staff. They redesign workflows. They stop contamination before it starts. If your facility doesn’t have a solid environmental monitoring program, you’re not just at risk of a recall. You’re gambling with people’s health. And that’s a risk no manufacturer should take.What’s the difference between environmental monitoring and product testing?
Product testing checks the final item-like a bottle of medicine or a packaged meal-for contamination. Environmental monitoring checks the surroundings: the air, floors, equipment, and surfaces that could contaminate the product during production. One tests the result; the other prevents the problem.
Do small manufacturers need environmental monitoring?
Yes. Even small facilities under 50 employees are legally required to monitor if they produce regulated products. The USDA found only 48% of small processors have fully compliant programs. That doesn’t mean they’re safe-it means they’re at high risk of failing inspections or causing outbreaks. Starting small is fine: focus on Zone 1 and 2, use ATP swabs, and train staff properly. You don’t need a $100,000 system to begin.
Why is Zone 3 so important if it’s not near the product?
Zone 3 includes things like forklifts, carts, and overhead pipes. These move between areas and can carry contaminants from Zone 4 into Zone 2 and 1. PPD Laboratories found that 62% of contamination events originated in Zone 3 or 4. Ignoring these areas is like ignoring the doorknob in a hospital room-you think it’s safe, but it’s how germs spread.
How long does it take to get results from environmental tests?
Traditional microbiological tests take 24-72 hours. That’s why many facilities use ATP testing for quick checks-it gives results in seconds. But ATP doesn’t identify the organism. For that, you still need lab culture. New methods like next-generation sequencing can cut pathogen ID time to under 24 hours, but they’re still expensive and not yet standard.
Can environmental monitoring prevent recalls?
Absolutely. The CDC estimates 87% of foodborne outbreaks linked to environmental contamination could have been prevented with proper monitoring. If you catch Listeria on a drain before it spreads to a slicer, you stop a recall before it starts. Monitoring doesn’t guarantee perfection-but it gives you control.

Jay Amparo
January 10, 2026 AT 21:22Man, I never thought about how much goes into making sure that jar of lotion doesn't turn into a germ hotel. Zone 3 is wild-people think it's just 'back there,' but those forklifts and pipes are basically contamination taxis. I work in a small pharma lab and we started tracking Zone 3 after a mold spike. Turned out it was condensation from an old pipe we'd ignored for years. Changed our whole cleaning schedule. Now we check it weekly. Game changer.
Also, ATP testing saved our bacon. No more waiting 72 hours to know if a surface is clean. We got our throughput up by nearly 40%. Still do lab tests for confirmation, but ATP? Non-negotiable now.
Lisa Cozad
January 12, 2026 AT 20:35So glad someone finally broke this down without sounding like a regulatory textbook. I work in a food plant with 32 employees and we were terrified of compliance until we started with Zone 1 and 2 only. ATP swabs, daily checks, and 10-minute training videos for new hires. No fancy software, no consultants. Just consistency.
And yeah-Zone 3 matters. We had a leaky ceiling above a cart storage area. Nobody cared until we found Listeria in three samples in a row. Turns out the water was dripping onto carts that got rolled into production. We fixed the roof. No more positives. Simple fix. Big win.
Saumya Roy Chaudhuri
January 14, 2026 AT 08:39Oh please. You people act like this is some groundbreaking revelation. I’ve been doing this since 2015. Zone 4? Quarterly? That’s amateur hour. Real facilities monitor Zone 4 daily with bioaerosol samplers and correlate with HVAC logs. And ATP? That’s a toy for people who don’t understand microbiology. It measures biomass, not pathogens. You could have a sterile surface with zero CFUs and still get a high ATP reading from dead skin cells. Pathogen-specific PCR? That’s what you need. But no, everyone wants the quick fix.
And don’t get me started on ‘training.’ 40 hours? Please. You need at least 80 hours with competency assessments, shadowing, and re-certification every 6 months. Or are you just trying to pass an audit and not actually prevent outbreaks?
Ian Cheung
January 16, 2026 AT 05:51Zone 3 is the silent killer and nobody talks about it enough. I once saw a guy roll a forklift from the break room into the packaging bay and nobody blinked. That thing had dust, crumbs, and who knows what else on the tires. We started tagging all carts with color-coded stickers-green for clean zones, red for high-risk zones. Now if someone rolls a red cart near Zone 1, someone yells. It’s dumb. It’s simple. It works.
Also ATP testing is the real MVP. I used to sit there staring at petri dishes like a wizard waiting for magic. Now I swipe, boom, green light, move on. No more 3-day delays. Product flies out the door. And yeah I know it doesn’t tell you what’s there-but I don’t need to know what it is if I know it’s clean enough to proceed. Save the DNA sequencing for the lab rats.
And if your data’s in 17 different spreadsheets you’re already losing. Get one platform. Even Excel with conditional formatting beats chaos.
anthony martinez
January 17, 2026 AT 10:18Wow. A whole article about something that’s been standard operating procedure since the 90s. And yet here we are, acting like it’s new. The FDA didn’t just wake up one day and say ‘hey let’s make everyone do this.’ We’ve had GMP since the 70s. People just don’t want to do the work. So they write blog posts about how ‘environmental monitoring is the invisible shield’ like it’s a Marvel movie.
Meanwhile, small manufacturers are still using Q-tips to swab surfaces and calling it ‘compliance.’
It’s not rocket science. It’s just… effort. And effort is hard.
Mario Bros
January 18, 2026 AT 21:21Big love to everyone putting in the work on this. Seriously. This stuff isn’t glamorous but it’s what keeps people safe. I’ve seen facilities where they just throw a swab on the floor and call it a day. Then they wonder why they got flagged. You wanna do this right? Train your people like they’re surgeons. Not interns.
And don’t sleep on data integration. I had a client who had 5 different logbooks. One for ATP, one for microbes, one for water, one for chemicals, one for maintenance. I told them to dump it all into one Google Sheet with color coding. Now they spot trends in minutes instead of weeks.
You’re not just checking boxes. You’re protecting real people. Keep going. You’re doing important work. 🙌
Jake Nunez
January 20, 2026 AT 09:28Just came back from a factory tour in Mumbai. Same principles, different scale. They don’t have fancy air samplers. They use cloth wipes and visual checks. But they have a culture of cleanliness that’s insane. Everyone wears gloves. No food allowed near production. Shoes are scrubbed at every door. And they rotate staff every 3 hours to avoid fatigue.
Turns out, you don’t need AI to prevent contamination. You need discipline. And respect for the process. The tech helps, but the mindset? That’s what matters. We could learn a lot from places where compliance isn’t enforced by regulators-it’s enforced by pride.
Christine Milne
January 21, 2026 AT 11:25While I appreciate the sentiment, this article demonstrates a profound misunderstanding of regulatory hierarchy. The FDA does not ‘treat it as a requirement’-it is codified under 21 CFR Part 110 and 211. The EU Annex 1 is not a ‘guidance’-it is legally binding under Regulation (EC) No 1223/2009 and 2001/83/EC. To frame this as ‘best practice’ is dangerously misleading.
Furthermore, the assertion that ‘monitoring is cheaper than failure’ is statistically inaccurate. The true cost of non-compliance includes criminal liability, corporate manslaughter charges, and permanent loss of market access-not just ‘recalls.’
This piece reads like marketing copy for a software vendor. Please consult the actual regulatory texts before publishing.
Bradford Beardall
January 21, 2026 AT 18:03Wait-so if Zone 3 is responsible for 62% of contamination events, why aren’t we talking about redesigning workflows to eliminate Zone 3 entirely? Like, why not just move all storage outside the production area? Or use automated guided vehicles that never enter anything but Zone 1? Why are we just accepting that ‘Zone 3 exists’ instead of asking how to make it obsolete?
I’ve seen plants where they built a separate corridor just for carts. No people allowed. No break rooms nearby. It cost $200k but cut contamination events by 90%. Maybe we’re thinking too small.
Also-next-gen sequencing? I’d love to see a cost-benefit breakdown. Is it worth it for a small plant? Or just for big pharma?
McCarthy Halverson
January 22, 2026 AT 05:00Start with Zone 1. Train your staff. Use ATP. Track trends. Fix what breaks. That’s it.
No need for fancy software. No need for 40-hour courses. Just do it right every time.
And stop ignoring Zone 3. It’s not ‘low risk.’ It’s ‘high opportunity for failure.’
Simple. Done.
Paul Bear
January 23, 2026 AT 22:51Let’s be precise here: ATP testing measures adenosine triphosphate, a molecule present in all living cells, which correlates with organic residue-not microbial load. Therefore, it is a hygiene indicator, not a microbiological one. To conflate ATP positivity with ‘clean enough’ is a fundamental misinterpretation of its purpose. The FDA’s 2023 draft guidance explicitly states that rapid methods must be validated against reference methods for equivalence. ATP does not identify pathogens. It does not replace culture. It is a screening tool. Period.
Additionally, the claim that ‘32% faster production turnarounds’ is misleading without context. Was this compared to facilities using no real-time feedback? Or compared to those using validated molecular diagnostics? The data is cherry-picked.
And please, stop calling Zone 4 ‘low risk.’ In immunocompromised populations, even ambient spores from Zone 4 can be lethal. Risk is not binary. It is probabilistic. And we owe it to patients to treat every surface with the gravity it deserves.
lisa Bajram
January 24, 2026 AT 03:48Y’all are underestimating the POWER of data integration!! I had a client who was drowning in spreadsheets-ATP here, microbial there, water quality over there, chemical logs in another file, maintenance logs in a binder, and the auditor showing up next week!! We threw it all into one dashboard-color-coded, auto-alerts, trend graphs, even a little ‘contamination heat map’-and guess what? They caught a recurring mold issue in Zone 2 because the system flagged a pattern: every time the AC ran on high, humidity spiked, and ATP readings jumped. They fixed the vent. Saved $1.2M in potential recalls.
Also-training videos? Film them yourself. Use your phone. Put them on a tablet by the door. Watch them before your shift. It’s not expensive. It’s just… intentional.
And if you think Zone 3 doesn’t matter… I’ve got a story about a forklift that carried salmonella from the dumpster to the packaging line. Don’t be that person. 💪🔥
Jaqueline santos bau
January 25, 2026 AT 11:09Okay but have you considered the emotional toll this takes on the workers? I’ve seen people cry because they got blamed for a contamination event they didn’t cause. They’re not trained properly. They’re rushed. They’re scared. And then the managers act like it’s just a ‘process’ and not a human being holding a swab at 3 AM.
And the ‘zone system’? It’s so rigid. What if your facility is a 100-year-old building with pipes running through everything? You can’t just slap a ‘Zone 3’ sticker on a leaky ceiling and pretend it’s solved.
Also-why is no one talking about the fact that 78% of contamination comes from people? Not surfaces. Not pipes. PEOPLE. We need to fix the culture, not just the swabs.
And I’ve seen managers ignore positive results because ‘it’s just one sample.’ That’s not data. That’s negligence. And I’m not mad. I’m just… disappointed.